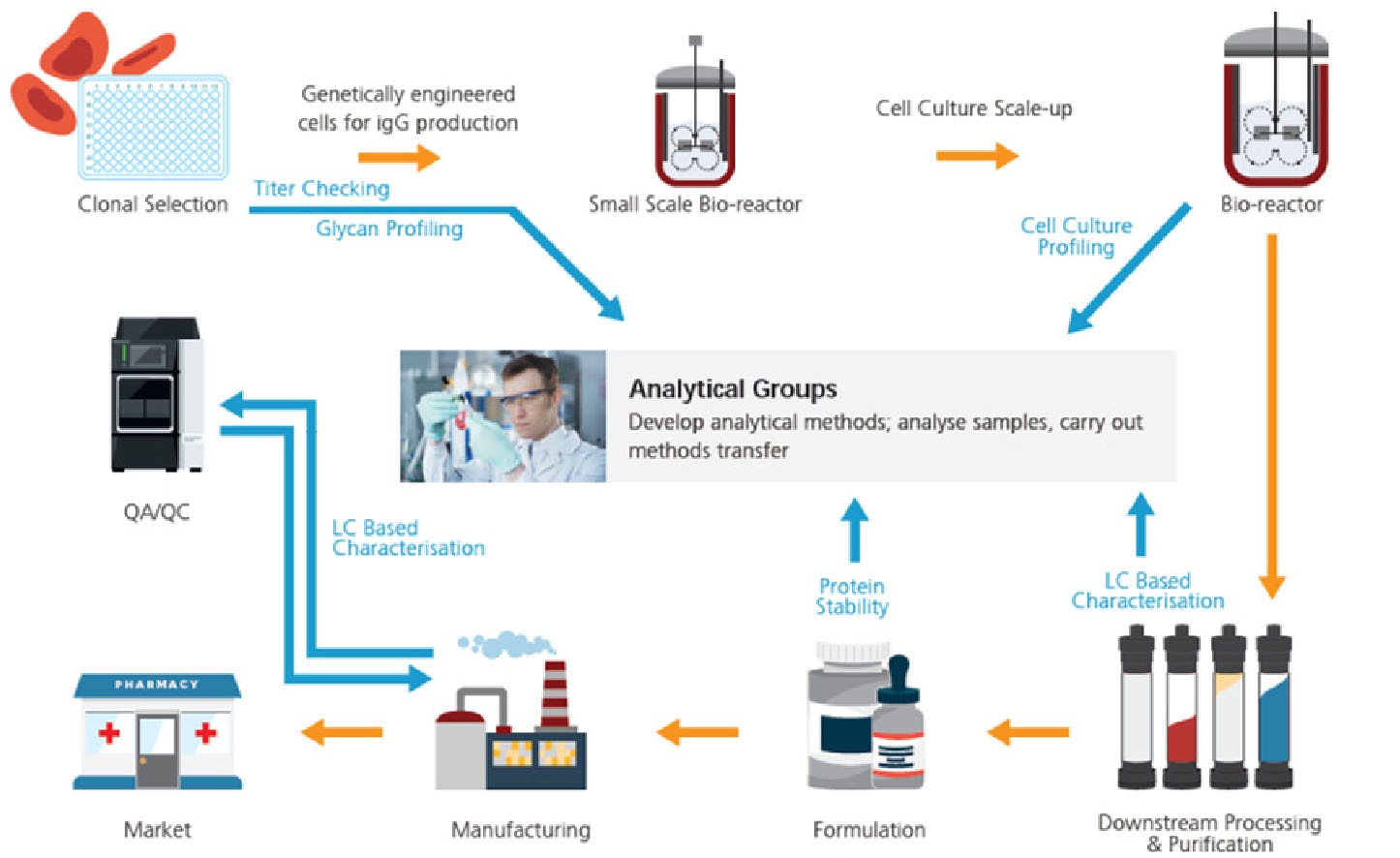
Achieving Fast, Robust, and Reliable Separation and Measurement in Biopharmaceuticals with Shimadzu's LC Columns

Biopharmaceuticals are medicines and drugs developed using biological means (e.g. in living cells). These drugs are generally high in molecular weight, unstable, immunogenic and possess complex and heterogeneous structures; these drugs include proteins, nucleic acids, and antibodies. With multiple stages in the biopharma workflow, industries must take into consideration various factors to optimize their strategy and operations. Ultimately, the safety and efficacy of the drug, and the speed and cost-efficiency of the entire workflow are key objectives of biopharma companies and the pharmaceutical industry.
To learn more, download Better Bioseparations with Shimadzu LC Columns.
The Use of Analytical Techniques in Biopharmaceuticals Analysis
There are several challenges faced by biopharma industries. To overcome them and seize the opportunities, industries must tap on various technologies to meet the biopharmaceuticals production and quality control standards, and also to accelerate their production process, including drug discovery, development, and compliance protocols. By employing advanced analytical techniques like chromatography, spectroscopy, and mass spectrometry, the highly regulated biopharma sector enhances its capabilities, ensuring the sustained benefit of healthcare systems and contributing to an improved quality of life for patients. This operational excellence not only fosters streamlined product innovation but also holds the potential to better patient outcomes for a range of diseases.
Liquid chromatography (LC) has been the technique of choice due to its relevance, wide applicability, and efficiency. With advancements in LC columns and instruments, LC analytical methods are shorter in analysis time, better in separation, and higher in signal intensity and sensitivity. In this brochure, you will learn more about the new Shimadzu LC columns and how they are applied in various biopharma workflows. Read on to understand how these LC columns, with Shimadzu LC and mass spectrometry (MS) work together to achieve fast, robust, and reliable separation and measurement.
Application Features: An Overview
Explore the applications of Shimadzu's LC columns in liquid chromatography and mass spectrometry, playing a key role in achieving rapid, robust, and reliable separation for accurate results. Uncover how these well-established techniques contribute to the development of biopharmaceuticals, the fastest-growing industry with promising potential.
Download the eBook to learn more or read on to get key insights.
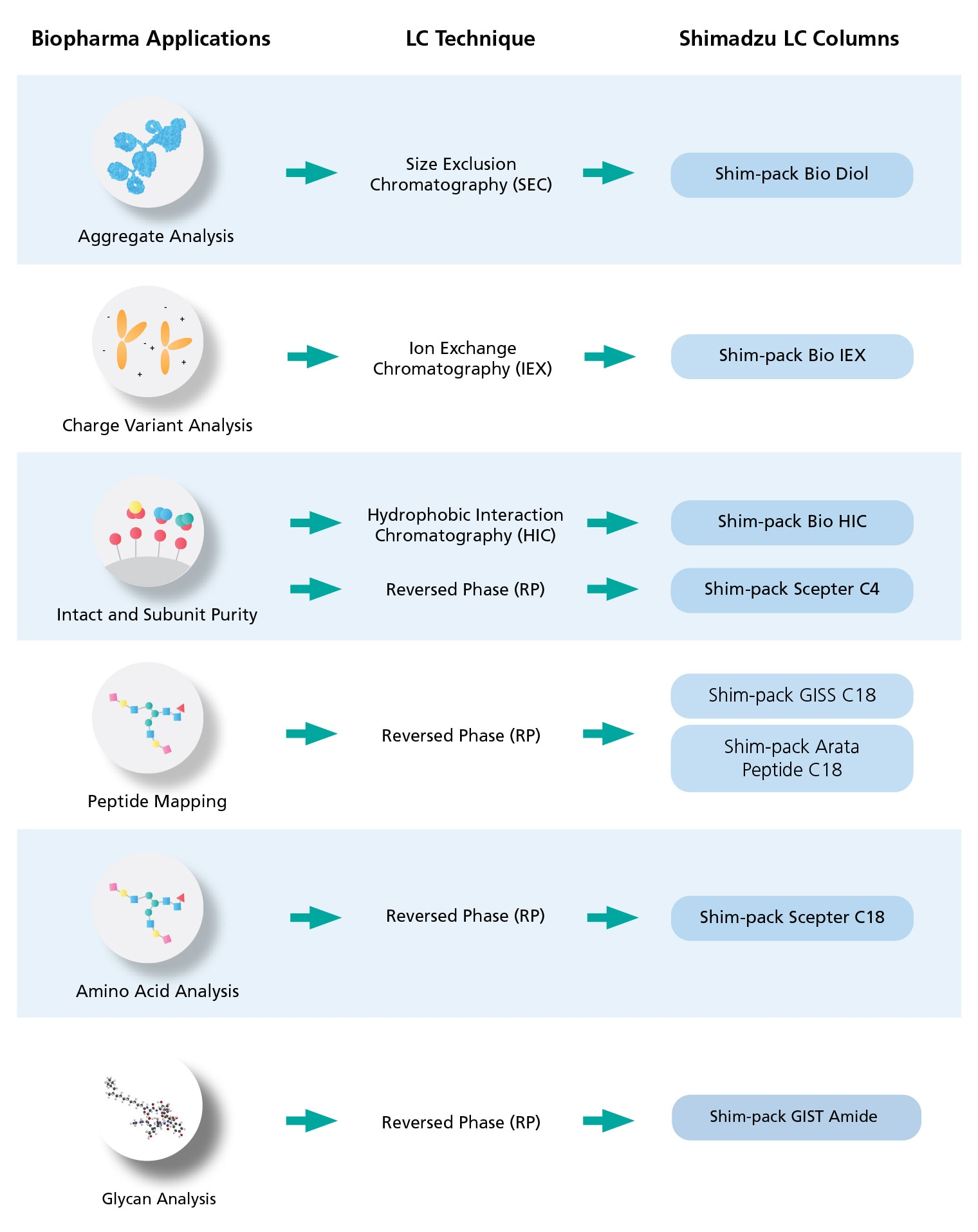
Size Exclusion Chromatography (SEC) for Aggregate and Fragment Analysis
SEC separates molecules by size and molecular weight. It has been the main technique for the characterization of protein-based therapeutic products (e.g. monoclonal antibodies, mAbs) due to its speed and reproducibility. As protein aggregation renders the drug to be unsafe and ineffective, the analysis of these undesirable protein aggregates and fragments could indicate the applicability and efficacy of the produced therapeutic drugs. Typically, SEC coupled to UV detection is used to analyze aggregation and conformational variants in order to monitor the integrity of the investigated drug sample.
Charge Variant Analysis using Ion Exchange Chromatography (IEX) Analysis
Proteins consist of many weak acidic and basic groups which can undergo changes in charge heterogeneity during biopharmaceutical production and purification processes. These not only affect the stability, but also the activity of the pharmaceuticals and may cause adverse immunological reactions. The identification of charge variants in development, and their monitoring throughout manufacturing, is critical to the production of safe and effective drugs. IEX utilizes the unique relationship between the net surface charge of the protein and pH for optimal protein separation. The pH or salt concentration defines the number of charges on the protein and helps to stabilize the native structure of the protein in the buffer used during analysis.
Intact Mass Analysis by Hydrophobic Interaction Chromatography (HIC)
HIC separates analytes based on its hydrophobic interactions with the column stationary phase. The separation occurs in less denaturing conditions and allows the proteins to maintain their biological activity, which is useful for protein separation and purification. For biopharma applications, HIC is suitable for the separation of antibody-drug conjugates (ADCs) with different drug-antibody ratios (DAR). Shim-pack Bio HIC columns can analyze DAR of ADC with relatively low pressure and high resolution.
Shim-Pack Scepter (Reversed Phase Column) for Intact mAbs Characterization
Monoclonal antibodies (mAbs) currently represent the largest class of therapeutic drugs made by the biopharma industry and will play a significant role in the future of pharmacological disease interventions. Purification, characterization and monitoring of mAbs are critically important to drug development, with a variety of analysis techniques routinely used.
Due to the heterogeneity in the hydrophobic structure of mAbs, reversed-phase LC separation is becoming a key choice for monitoring purity and stability during manufacturing, formulation and storage. Shimadzu offers reliable solutions for identifying and quantifying degradation products, and monitoring them throughout production and formulation, using LC systems, and MS for quantitation, and/or high-resolution accurate mass analysis.
Tackle Peptide Mapping with Reversed Phase Chromatography
Comprehensive protein characterization is crucial for the biotherapeutics quality control, process monitoring, and more. It involves the detection and monitoring of single amino acid changes, modifications, and degradation products. Peptide mapping is an essential technique for studying the primary structure of proteins and it typically involves enzymatic digestion of protein, followed by chromatographic separation. This technique has become much more convenient and used as a method of choice in many biopharma analysis and workflows.
Shimadzu offers a comprehensive portfolio of solutions for the highly accurate confirmation of protein sequence, identification of modifications, and routine protein fingerprint monitoring for QA/QC, using LC systems and columns, LC-MS systems and MALDI-MS platforms.
For full details, access the comprehensive eBook below.
Get Better Bioseparations With Shimadzu LC Columns
As the biopharma industry collectively strives to bring pharmaceutical products to market, key objectives include ensuring the safety and efficacy of the developed drugs, as well as enhancing speed and reducing operating costs of the entire workflow. This encompasses various stages, including clinical development, where precise and sensitive analytical technology comes into play to support with innovative solutions that address the complexities of drug development, with product quality and efficiency in mind.
Get better bioseparations for biopharmaceutical analysis below.
