Shimadzu’s Analysis Solutions for Pharmaceutical Packaging, from Physical Strength Evaluation to Quality Control

Shimadzu’s Analysis Solutions For Pharmaceutical Packaging
Read on below to learn more and get a few bonus resources!
In the development and quality control of pharmaceuticals and pharmaceutical packaging, it is necessary to ensure their efficacy and safety. Therefore, various evaluations are performed. For example, tablets must be hard enough to not crack or split during their manufacture and transport. But if they are too hard, their solubility when swallowed could be reduced, so they must be manufactured to the appropriate hardness.
Pharmaceutical packaging, also known as drug packaging, encompasses the materials used to encase medications for the purposes of distribution, storage, and consumption. By safeguarding medications against external factors such as contaminants, moisture, damage, and tampering, pharmaceutical packaging plays a pivotal role in maintaining the safety and quality of pharmaceutical products throughout their shelf life. Classified into primary packaging, secondary packaging and tertiary packaging, pharmaceutical packaging exists in diverse materials and compositions, such as in blister packaging, sachet packaging and even glass bottles like vials and ampoules.
For example, the packaging of pharmaceuticals must also protect them from moisture and light so that their quality does not deteriorate. Typically, pharmaceuticals are enclosed in plastic or aluminum using PTP sheets, and the packaging is designed to dispense them by pressing the sheet. Therefore, the packaging must be manufactured so that the force to push them out is appropriate. Testing machines are used to perform these evaluations.
To ensure adherence to regulatory standards and uphold product quality, rigorous quality control measures are frequently employed to evaluate the physical integrity of pharmaceutical packaging and products.
These assessments utilize a range of testing methods, including the pill compression test, pellet crush test, and press-dispense test, upholding good practice for maintaining the safety, standardization, and durability of pharmaceutical packaging and products.
They evaluate various packaging designs that accommodate diverse medication delivery methods, encompassing tablets, capsules, liquid drugs, parenteral drugs, and more.
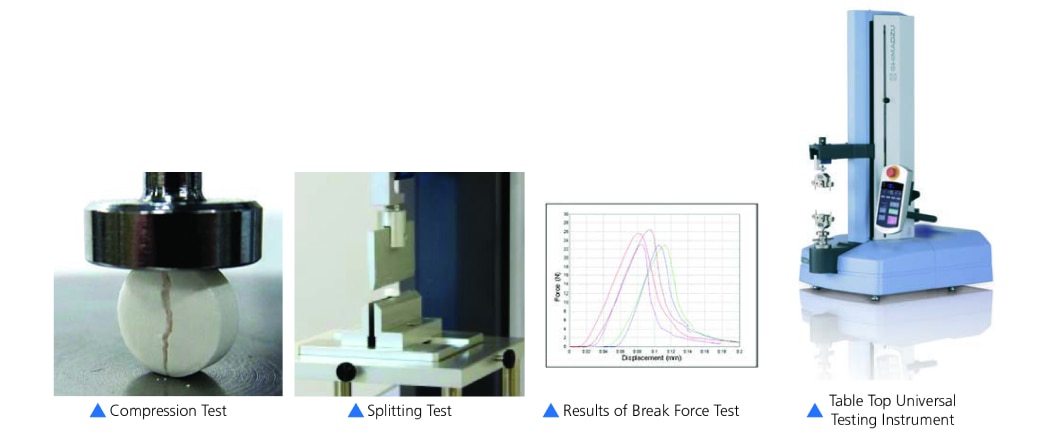
Pill Compression Test
- Pill crush test is performed in order to understand the compressive properties of tablets. Evaluate in terms of hardness, powder molding, and surface coating characteristics.
- Splitting test is performed on tablets with grooves in the center, allowing each to be taken whole by adults or in a half dose by children.
- For this reason, it must be split in half using some suitable degree of force.
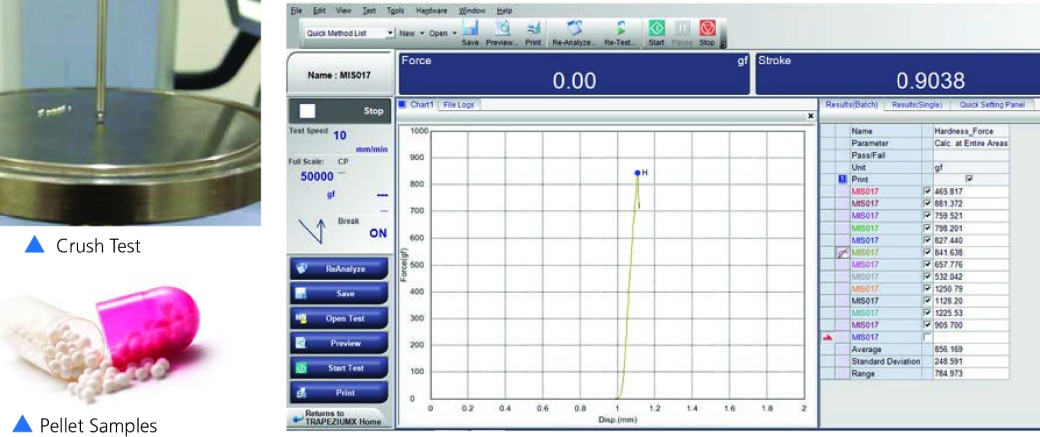
Pellet Crush Test
- The objective of testing is to measure the crushing strength of pellets at which failure occurs.
- The peek force i.e., Force required to crush the pellet coating is marked on the graph and readings are recorded in the software. Refer here.
- Our testing and observation: When 10 gm pre-load force is detected, probe further crush the sample to measure the maximum peak and stop the test once the load drops to the 80% of maximum hardness value.
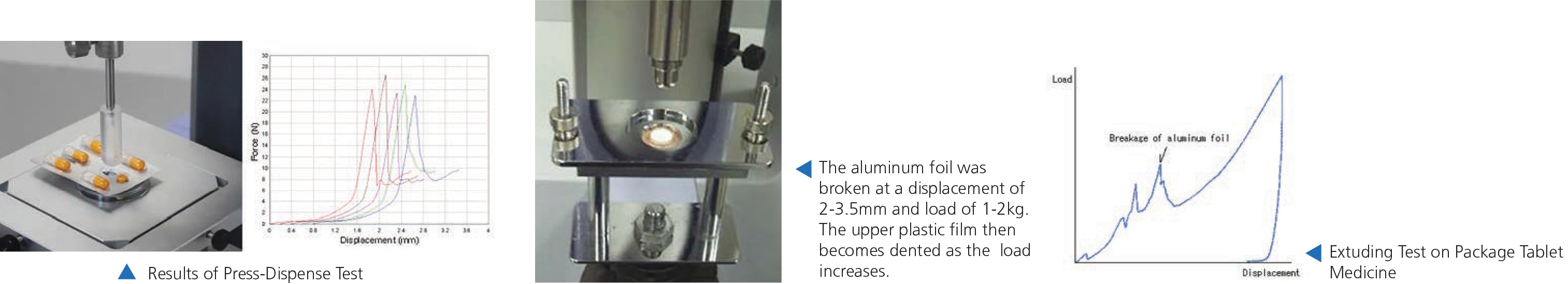
Press-Dispense Test for PTP Packaged Capsules
- Quality control is necessary to ensure the package does not peel excessively, and the capsule is not too difficult to dispense.
- Control the ease of capsule extraction by evaluating the max force of PTP which is applicable to product development and quality control.
Get a Copy of the Physical Strength Evaluation of Pharma Products & Packaging Here
Analysis of Extractables from Pharmaceutical Packaging
Mutual interactions between the pharmaceutical and packaging are a problem with pharmaceutical packaging materials that require particular care. Extractables are compounds generated from storage conditions that are more severe than normal, whereas leachables are compounds that can be transferred from the packaging to the pharmaceutical under normal storage conditions. They are classified as indicated in Table 1. Before pharmaceuticals can be sold, extractables and leachables must be comprehensively verified to determine packaging material risks.
| Extractables | Leachables | |
|---|---|---|
| Description | All compounds that potentially could be extracted from packaging | Compounds that leach out under normal usage conditions |
| Extraction Conditions | More severe conditions than for normal use or storage. High-temperature extraction or solvent extraction is used. | Under normal conditions for use or storage. |
| Measured Item | Packaging material | Pharmaceutical |
The FDA summarized the potential interactions between pharmaceuticals and packaging materials in “Guidance for Industry: Container Closure Systems for Packaging Human Drugs and Biologics.” That guidance indicates an increased danger to humans from parenterally administered drugs or parenterally administrable drug suspensions (Table 2).
|
Degree of Concern Associated with the Route of Administration
|
Likelihood of Packaging Component-Dosage Form InteractionTitle | ||
|---|---|---|---|
| High | Medium | Low | |
| Highest | Inhalation Aerosols and Solutions; Injections and Injectable Suspensions | Sterile Powders and Powders for Injection; Inhalation Powders | |
| High | Ophthalmic Solutions and Suspensions; Transdermal Ointments and Patches; Nasal Aerosols and Sprays | ||
| Low | Topical Solutions and Suspensions; Topical and Lingual Aerosols; Oral Solutions and Suspensions | Topical Powders; Oral powders | Oral Tablets and Oral (Hard and Soft Gelatin) Capsules |
Therefore, in addition to strength evaluation of pharmaceutical packaging, the analysis of extractables from the same material is important to ensure drug safety. Extractables can be identified by both solvent and high-temperature extraction methods. By using a variety of solvents, solvent extraction can be used for compounds with a wide range of properties. On the other hand, using a headspace sampler with high-temperature extraction can enable samples to be pretreated more simply and quickly than for solvent extraction.
Click Here For Details on the Analytical Methods in Analysis
Watch Our Webinar on Pharmaceutical Packaging
With the focus on delivering patient-centric administration for better treatment adherence, there is a demand to make drugs easier to handle and swallow. Similarly, packaging materials need to be more practical and safer to avoid unintended interactions, especially with biotherapeutics, which are labile and would result in compromise on the drug efficacy.
Watch our webinar to discover how you can ensure the quality of pharmaceutical products and packaging with Shimadzu – with compact and high-performing machines, we offer rapid thermal analysis and strength testing for evaluations to achieve optimization in these designs.
