Antibody Therapeutics: A Guide To What’s New And Next
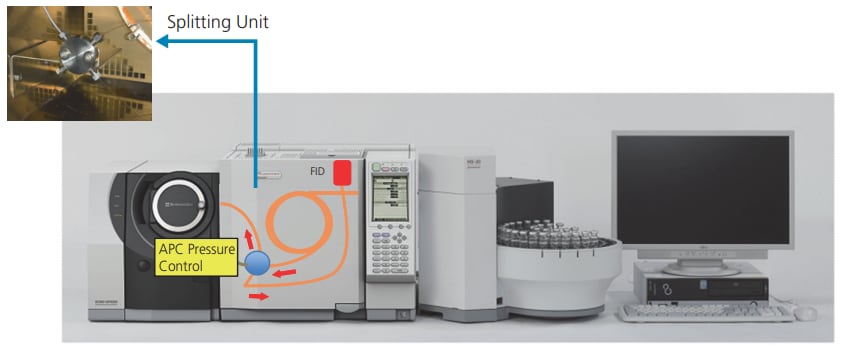
Analysis of Residual Solvents in Pharmaceuticals Using Headspace GC-FID/MS Detector Splitting System
Headspace gas chromatography with flame ionization detection (GC-FID) is often used for residual solvent testing of pharmaceuticals, though the qualitative power of this method is not particularly high. Because gas chromatography mass spectrometry (GC/MS) utilizes MS to perform qualitative analysis based on mass spectra, GC/MS can be used to estimate and identify individual peaks detected in the expected vicinity of a target solvent as well as other unknown peaks.
We describe an example of residual solvent test of a pharmaceutical using a detector splitting system that simultaneously obtains FID and MS data in a single measurement.

GCMS-QP2020 NX
The GCMS-QP2020 can assist any laboratory, regardless of its analysis focus, achieve its full potential.
News & Events
-
-
Nitrosamines/NDSRI In Focus: New Analytical Insights And Latest Solutions
-
Stay Ahead In Pharma: Download The Expert eBook Now!
-
From Sample To Results: Complete Solutions For Oligonucleotide Analysis
-
This Is The Power Of AI - Discover LabSolutions MD Now
-
Introducing Nexera FV: Discover Its Leading-Edge Capabilities For Pharma